Second year CSCT student, Jemma Rowlandson, writes about her research topic of materials for hydrogen storage. Jemma recently won the regional finals of the Institution of Engineering and Technology's Present Around the World Competition and won a prize of £300 and a place at the national finals.
One of the greatest challenges faced by our generation is global warming. As global temperatures continue to rise, this will lead to severe and potentially irreversible climate change. The big question is, how do we stop it?
 Transport accounts for a quarter of domestic carbon dioxide emissions in the UK. Not only this, but vehicles produce particles which lower the air quality and can be harmful. This is why a lot of research in the CSCT and elsewhere focuses on replacing diesel and petrol cars. One potential technology we could use is hydrogen.
Transport accounts for a quarter of domestic carbon dioxide emissions in the UK. Not only this, but vehicles produce particles which lower the air quality and can be harmful. This is why a lot of research in the CSCT and elsewhere focuses on replacing diesel and petrol cars. One potential technology we could use is hydrogen.
Hydrogen is the most lightweight and abundant element in the universe, and it could be the answer to a lot of our problems. Hydrogen is used as rocket fuel, and with good reason; it has a very high energy density, meaning you need to use a lot less of it in comparison to petrol or diesel. Not only this, but hydrogen has the amazing potential to be completely green. This is because you can make hydrogen by splitting water, use that hydrogen to power your car, and out of the exhaust comes only water!
Although this seems like a perfect solution, there are a couple of very big problems associated with hydrogen technology. One of the most critical is that hydrogen is a gas and so very difficult to store, because it takes up a lot space. To store 4 kg of hydrogen at room temperature and atmospheric pressure (enough to get you from Manchester to London) you would need to attach about 600 party balloons full of flammable hydrogen gas to your car. Not a great idea.
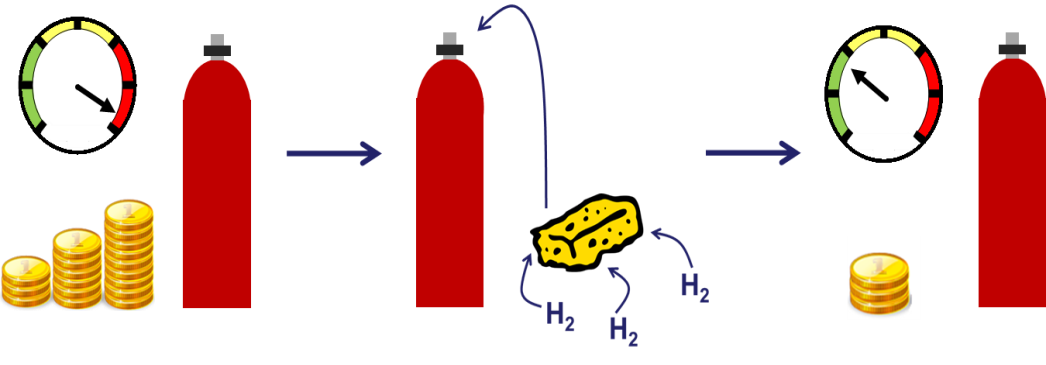
So what can we do instead? Well the best way at the moment is to compress the hydrogen into a gas cylinder, at either 350 or 700 times atmospheric pressure. This in turn comes with its own problems. For a start not everyone is entirely comfortable sitting above a highly pressurised flammable hydrogen gas cylinder. The other is that this is actually very expensive! You’ve not only got the energy cost of compressing the gas, but also the cost of the cylinder itself which has to be able to withstand a car crash. If we ever want to see mass market hydrogen cars we need to drop the price of this fuel tank.
There are many different approaches to hydrogen storage; the one focused on at Bath is to use a nanoporous material. There are lots of materials to choose from but they all work in pretty much the same way, using a process called adsorption. Now this is different to absorption, which is the process of taking something in (like a sponge absorbs water). Adsorption by contrast is when something sticks or ‘adsorbs’ onto a surface. For hydrogen storage this means the hydrogen gas molecules stick to the surface of the material, packing closely together and increasing your hydrogen storage capacity. If you put this material inside a gas cylinder you could store the same amount of hydrogen but at a lower pressure, making it both safer and cheaper.
Related Post:
Jemma Rowlandson wins the local round of the IET PATW
Respond